Sunday, June 30, 2013
Blog Question
Explain how the solubility curve lab works.
The solubility lab works by testing specific solutes and seeing how they react with water or other solvents. This lab is to see what it takes for a solute to dissolve in a specific solvent. And by my observation, I will decide whether to classify the solute as soluble, slightly soluble, or insoluble at room temperature water.
The solubility lab works by testing specific solutes and seeing how they react with water or other solvents. This lab is to see what it takes for a solute to dissolve in a specific solvent. And by my observation, I will decide whether to classify the solute as soluble, slightly soluble, or insoluble at room temperature water.
ISCS (p.82-83) #9-19
6/30/13
9.
-55 x .20 = 11g (mass of sugar)
-55 - 11 = 44g (mass of water)
10.
-0.015 x 1,000,000 = 15,000 ppm
11. What makes water molecules polar is the end that contains the oxygen is negative and the end that contains the hydrogen is positive; therefore, making an attraction that forms H2O.
12. How molecules in liquid water arrange themselves relative to one another:
13.
a. oxygen molecule: O-
b. hydrogen molecule: H+
14. Heavy metals are called "heavy" because their atoms have greater masses than those of essential metallic elements, and are therefore harmful to humans and other organisms.
15. Three symptoms of heavy- metaled poisoning include damages to the nervous system, brain, kidney, and liver.
16.
a. Two possible human exposures to lead include paint/pottery and the transportation of water through lead pipes.
b. Two possible human exposures to mercury include medical and weather thermometers and fluorescent light bulbs.
17. The ion that can be found in many bases is the hydroxide ion (OH-).
18. The element hydrogen (H) is found in most acids.
19.
a. seawater: basic
b. drain cleaner: basic
c. vinegar: acidic
d. pure water: neutral
ISCS (p.83) #20-27, 33, 35
20.
a. soft drinks
b. black coffee
c. milk of magnesia
21. A solution at pH 2.0 is 20 times more acidic than a solution at pH 4.0.
22. Three negative effects of inappropriate pH levels on aquatic organisms are:
- Too low pH (acidic)
- fish egg development is impaired causing reproduction to be more difficult
- increased concentrations of metal ions in natural waters by the leakage of metal ions from surrounding soil
- Too high pH (basic)
- dissolves organic material like skin on humans or scales on fish
23. Polar molecules have different charges at each end. One end is positive and one end is negative. Nonpolar molecules are balanced at both ends, they don't have these different charges. Polar molecules dissolve in polar solvents and nonpolar molecules dissolve in nonpolar solvents.
24. I would pick lamp oil to dissolve a nonpolar molecular substance because oil is a non polar solvent, and since nonpolar molecules dissolve in nonpolar solvents, the nonpolar molecular substance would; therefore, dissolve in lamp oil better than in water and ethanol sine these two are polar molecules.
25. Table salt (NaCl) dissolves in water but not cooking oil because water is polar and can, then, attach to the positive and negative charges of NaCl, causing the salt to pulled apart and dissolve in water. And since cooking oil is nonpolar, it doesn't have this same effect.
26. The term "like dissolves like" refers to pattern of solubility behavior, which is polar substances dissolve in polar solvents, and nonpolar substances dissolve in nonpolar solvents. Therefore, a polar substances will not dissolve in a nonpolar solvent, and likewise if a nonpolar substance tries dissolving in a polar solvent.
27. You cannot satisfactorily clean greasy dishes with just plain water because greasy dishes contain oil which is nonpolar, and water is polar. And because "like dissolves like" a nonpolar substance cannot be dissolved in a polar solvent. Which is why you need to add soap to the water to help dissolve the substances.
33.
a. The kind of materials that are likely to be found in these waterless hand cleaners would be nonpolar substances.
b. Using these cleaners would be more effective than washing with water because the greese, which is nonpolar, cant be dissolved by water, which is polar. So these cleaners are capable of cleaning the grease because they are nonpolar.
35. In HF, I would expect hydrogen (H) to have a particle positive charge. This is because fluorine (F) has the greatest electronegativity of any element, meaning it has a higher concentration of electrons, which are negative. Which means hydrogen would have to be positive if the fluorine (F) is a particle negative charge.
9.
-55 x .20 = 11g (mass of sugar)
-55 - 11 = 44g (mass of water)
10.
-0.015 x 1,000,000 = 15,000 ppm
11. What makes water molecules polar is the end that contains the oxygen is negative and the end that contains the hydrogen is positive; therefore, making an attraction that forms H2O.
12. How molecules in liquid water arrange themselves relative to one another:
13.
a. oxygen molecule: O-
b. hydrogen molecule: H+
14. Heavy metals are called "heavy" because their atoms have greater masses than those of essential metallic elements, and are therefore harmful to humans and other organisms.
15. Three symptoms of heavy- metaled poisoning include damages to the nervous system, brain, kidney, and liver.
16.
a. Two possible human exposures to lead include paint/pottery and the transportation of water through lead pipes.
b. Two possible human exposures to mercury include medical and weather thermometers and fluorescent light bulbs.
17. The ion that can be found in many bases is the hydroxide ion (OH-).
18. The element hydrogen (H) is found in most acids.
19.
a. seawater: basic
b. drain cleaner: basic
c. vinegar: acidic
d. pure water: neutral
ISCS (p.83) #20-27, 33, 35
20.
a. soft drinks
b. black coffee
c. milk of magnesia
21. A solution at pH 2.0 is 20 times more acidic than a solution at pH 4.0.
22. Three negative effects of inappropriate pH levels on aquatic organisms are:
- Too low pH (acidic)
- fish egg development is impaired causing reproduction to be more difficult
- increased concentrations of metal ions in natural waters by the leakage of metal ions from surrounding soil
- Too high pH (basic)
- dissolves organic material like skin on humans or scales on fish
23. Polar molecules have different charges at each end. One end is positive and one end is negative. Nonpolar molecules are balanced at both ends, they don't have these different charges. Polar molecules dissolve in polar solvents and nonpolar molecules dissolve in nonpolar solvents.
24. I would pick lamp oil to dissolve a nonpolar molecular substance because oil is a non polar solvent, and since nonpolar molecules dissolve in nonpolar solvents, the nonpolar molecular substance would; therefore, dissolve in lamp oil better than in water and ethanol sine these two are polar molecules.
25. Table salt (NaCl) dissolves in water but not cooking oil because water is polar and can, then, attach to the positive and negative charges of NaCl, causing the salt to pulled apart and dissolve in water. And since cooking oil is nonpolar, it doesn't have this same effect.
26. The term "like dissolves like" refers to pattern of solubility behavior, which is polar substances dissolve in polar solvents, and nonpolar substances dissolve in nonpolar solvents. Therefore, a polar substances will not dissolve in a nonpolar solvent, and likewise if a nonpolar substance tries dissolving in a polar solvent.
27. You cannot satisfactorily clean greasy dishes with just plain water because greasy dishes contain oil which is nonpolar, and water is polar. And because "like dissolves like" a nonpolar substance cannot be dissolved in a polar solvent. Which is why you need to add soap to the water to help dissolve the substances.
33.
a. The kind of materials that are likely to be found in these waterless hand cleaners would be nonpolar substances.
b. Using these cleaners would be more effective than washing with water because the greese, which is nonpolar, cant be dissolved by water, which is polar. So these cleaners are capable of cleaning the grease because they are nonpolar.
35. In HF, I would expect hydrogen (H) to have a particle positive charge. This is because fluorine (F) has the greatest electronegativity of any element, meaning it has a higher concentration of electrons, which are negative. Which means hydrogen would have to be positive if the fluorine (F) is a particle negative charge.
Thursday, June 27, 2013
Blog Question
Explain how to do solution concentration problems. What is the tricky part?
How you do solve solution concentration problems is by taking the mass of the solute (small number) and divide it by the mass of the solution (total number), and then multiply it by 100. This is the tricky part, which is calculating the percent of mass.
How you do solve solution concentration problems is by taking the mass of the solute (small number) and divide it by the mass of the solution (total number), and then multiply it by 100. This is the tricky part, which is calculating the percent of mass.
C.5 (p.62) #1-3
6/27/13
1.
a. The changes I would see in the beaker if I dissolved 40g KCl at 50'C and then let it cool to room temperature at 25'C would be a supersaturated solution. Because at first the solution was unsaturated, and when it cooled, it became supersaturated because the solution contained more KCl than it can contain at a lower temperature.
b.
2.
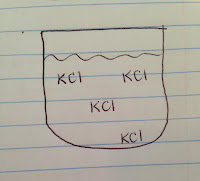
a. Solution made by dissolving 20g KCl in 100g water at 40'C:
b.
i. Model of a solution that is kept at 40'C with one- fourth of the water evaporated. This solution differs from the original solution because it is more concentrated since water was taken out and not equally distributed.
ii. 20g of water must evaporate at this temperature to create a saturated solution.
3.
a. Solution containing 10g KCl in 100g water at 25'C.
b. Dilute the solution by adding 100g of water.
c. The key features that are different in questions 3a and 3b is that in 3b the amount of water added was doubled making the solution's concentration less than that in 3a. Therefore, making the potassium chloride (KCl) in 3b more spread out.
1.
a. The changes I would see in the beaker if I dissolved 40g KCl at 50'C and then let it cool to room temperature at 25'C would be a supersaturated solution. Because at first the solution was unsaturated, and when it cooled, it became supersaturated because the solution contained more KCl than it can contain at a lower temperature.
b.
2.
a. Solution made by dissolving 20g KCl in 100g water at 40'C:
i. Model of a solution that is kept at 40'C with one- fourth of the water evaporated. This solution differs from the original solution because it is more concentrated since water was taken out and not equally distributed.
ii. 20g of water must evaporate at this temperature to create a saturated solution.
3.
a. Solution containing 10g KCl in 100g water at 25'C.
b. Dilute the solution by adding 100g of water.
c. The key features that are different in questions 3a and 3b is that in 3b the amount of water added was doubled making the solution's concentration less than that in 3a. Therefore, making the potassium chloride (KCl) in 3b more spread out.
Wednesday, June 26, 2013
Blog Question
Explain your way of solving math word problems when answering solubility questions.
By understanding the chart on p.54, I was able to solve math word problems when answering solubility questions. And once I understood the graph, all I had to do is follow the lines given to me to see at what temperature, or how many grams can dissolve a solute in 100g of water.
By understanding the chart on p.54, I was able to solve math word problems when answering solubility questions. And once I understood the graph, all I had to do is follow the lines given to me to see at what temperature, or how many grams can dissolve a solute in 100g of water.
C.2 (p.56) #1-3
6/26/13
1.
a. The mass (grams) of potassium nitrate (KNO3) that will dissolve in 100g water at 60'C is 108g.
b. The mass (grams) of potassium chloride that will dissolve in 100g water at 60'C is 44g.
2.
a. 20g of potassium nitrate can be added to form a saturated solution at 30'C.
b. 45g is the minimum mass of 30'C water needed to dissolved 25g potassium nitrate.
3.
a. If the solution is agitated, than 90g of potassium nitrate will precipitate.
b. To dissolve all of the KNO3, you would have to add 110 of the 55'C water.
ISCS (p.82) #1-8
1. Three teaspoons of sugar will completely dissolve in a serving of hot tea, and not dissolve in the equally sized serving of hot tea is because different temperatures can affect solubility. So and when temperatures are higher, the solute dissolves more easily.
2. The maximum mass of potassium chloride that will dissolve is 48g.
3.
a. 100.0 mL water: 200 grams
b. 355 mL water: 710 grams
c. 946 mL water: 1,892 grams
4.
a. NaCl, KCl, KNO3
b. KNO3, KCl, NaCl
5. Saturated is when a solvent has dissolved as much solute as it can retain at a specific temperature, and therefore the solute settles at the bottom of the container; even stirring the mixture will not make the crystals dissolve. Unsaturated is a solution that contains less dissolved solute than the amount that the solvent can normally hold at a specific temperature.
6. Solutibility of potassium nitrate (KNO3)
a. 30 grams
b. Supersaturated
c. 90 grams
7.
a. Unsaturated: the crystal will dissolve, therefore possibly making the solution saturated
b. Saturated: the crystal will settle to the bottom as a precipitate because the solvent has already dissolved as much solute as it can.
c. Supersaturated: the crystal will settle to the bottom of the container as a precipitate, and by doing this it will add on to the precipitate that was already there.
8. The percent concentration of ethanol, expressed as percent ethanol by mass is 1.15 x 35 = 40.25%.
1.
a. The mass (grams) of potassium nitrate (KNO3) that will dissolve in 100g water at 60'C is 108g.
b. The mass (grams) of potassium chloride that will dissolve in 100g water at 60'C is 44g.
2.
a. 20g of potassium nitrate can be added to form a saturated solution at 30'C.
b. 45g is the minimum mass of 30'C water needed to dissolved 25g potassium nitrate.
3.
a. If the solution is agitated, than 90g of potassium nitrate will precipitate.
b. To dissolve all of the KNO3, you would have to add 110 of the 55'C water.
ISCS (p.82) #1-8
1. Three teaspoons of sugar will completely dissolve in a serving of hot tea, and not dissolve in the equally sized serving of hot tea is because different temperatures can affect solubility. So and when temperatures are higher, the solute dissolves more easily.
2. The maximum mass of potassium chloride that will dissolve is 48g.
3.
a. 100.0 mL water: 200 grams
b. 355 mL water: 710 grams
c. 946 mL water: 1,892 grams
4.
a. NaCl, KCl, KNO3
b. KNO3, KCl, NaCl
5. Saturated is when a solvent has dissolved as much solute as it can retain at a specific temperature, and therefore the solute settles at the bottom of the container; even stirring the mixture will not make the crystals dissolve. Unsaturated is a solution that contains less dissolved solute than the amount that the solvent can normally hold at a specific temperature.
6. Solutibility of potassium nitrate (KNO3)
a. 30 grams
b. Supersaturated
c. 90 grams
7.
a. Unsaturated: the crystal will dissolve, therefore possibly making the solution saturated
b. Saturated: the crystal will settle to the bottom as a precipitate because the solvent has already dissolved as much solute as it can.
c. Supersaturated: the crystal will settle to the bottom of the container as a precipitate, and by doing this it will add on to the precipitate that was already there.
8. The percent concentration of ethanol, expressed as percent ethanol by mass is 1.15 x 35 = 40.25%.
Tuesday, June 25, 2013
Blog Question
What did you learn from this lab about water and about process?
From this lab I learned that water isn't 100% pure because there are hidden electrical charges, called ions, inside water. I learned that ions are not visible to humans, but by preforming confirming tests ions can, then, be seen by humans. From this lab I also learned a lot about ions. I also learned that it is essential to wash all equipment before preforming a new test because substances from one test can have a big impact on another test.
ISBS (p.51-52) #25-34
6/25/13
25. Qualitative tests have to do with the qualities of substance, these tests identify the presence or absence of a particular substance in a sample. Quantitative tests determine the amount of a specific substance is present in a sample.
26. A confirming test is a test that confirms ions are present.
27.
a) A reference solution is a solution of known components. The purpose of the reference solution is to have a solution where you can compare your other solutions to.
b) The purpose of a distilled water bank is it is a bank known not to contain any ions of interest; therefore, when an ion solution is added to it, there is no reaction. It acts as another comparison solution.
28. If a student tests for iron and observes no water change, then student should not conclude that no iron is present because sometimes when there is a negative test that may either mean there is no ion present, or the ion in present in such low amounts; therefore, it is not certain there is an accurate result.
29.
a) Given an un known mixture, the steps I would follow to classify it as a solution, a suspension, or a colloid would be first mixing the mixture and allowing it to sit for a while. By doing this I can see if particles settle to the bottom of the container. Another way I would classify the mixture would be by using the Tyndall effect.
b) These steps would help me distinguish between the three mixtures by allowing me to see if particles settle to the bottom of the solution after it is mixed and is allowed to settle for a minute. If the particles separate and collect to the bottom, it would show a suspension. If the mixture has a positive Tyndall effect (scattering of light caused by the suspension of particles), then it is a colloid. And if neither of these techniques work, then it is a solution.
30. Failing to follow the instructions "shake before using" on a label of a medicine bottle would be a risk because most of the large particles are on the bottom of the bottle, and are not eventually distributed within the bottle. Therefore, you must shake the bottle to mix the large particles and the liquid together. If you fail to do this you may not be taking the actual medicine part of the mixture, meaning your medication may not work to the best of its ability.
31. It is useful for element symbols to have international acceptance because it allows scientist from all over the world to be able to study the same elements.
32. A model of a solution where water is the solvent and oxygen gas is the solute:
33. No. It is possible to have 100% "chemical free" water because it is impossible to have completely pure water when the atmospheric gases, nitrogen, oxygen, and carbon dioxide, always dissolve in water. Even with the expensive process of distillation, it is still impossible to have 100% "chemical free" water.
34. Water is composed of two hydrogen atoms and one oxygen atom and water's physical property is a liquid. However, the elements that make up water are not a liquid. The physical property of both hydrogen and oxygen is a gas.
25. Qualitative tests have to do with the qualities of substance, these tests identify the presence or absence of a particular substance in a sample. Quantitative tests determine the amount of a specific substance is present in a sample.
26. A confirming test is a test that confirms ions are present.
27.
a) A reference solution is a solution of known components. The purpose of the reference solution is to have a solution where you can compare your other solutions to.
b) The purpose of a distilled water bank is it is a bank known not to contain any ions of interest; therefore, when an ion solution is added to it, there is no reaction. It acts as another comparison solution.
28. If a student tests for iron and observes no water change, then student should not conclude that no iron is present because sometimes when there is a negative test that may either mean there is no ion present, or the ion in present in such low amounts; therefore, it is not certain there is an accurate result.
29.
a) Given an un known mixture, the steps I would follow to classify it as a solution, a suspension, or a colloid would be first mixing the mixture and allowing it to sit for a while. By doing this I can see if particles settle to the bottom of the container. Another way I would classify the mixture would be by using the Tyndall effect.
b) These steps would help me distinguish between the three mixtures by allowing me to see if particles settle to the bottom of the solution after it is mixed and is allowed to settle for a minute. If the particles separate and collect to the bottom, it would show a suspension. If the mixture has a positive Tyndall effect (scattering of light caused by the suspension of particles), then it is a colloid. And if neither of these techniques work, then it is a solution.
30. Failing to follow the instructions "shake before using" on a label of a medicine bottle would be a risk because most of the large particles are on the bottom of the bottle, and are not eventually distributed within the bottle. Therefore, you must shake the bottle to mix the large particles and the liquid together. If you fail to do this you may not be taking the actual medicine part of the mixture, meaning your medication may not work to the best of its ability.
31. It is useful for element symbols to have international acceptance because it allows scientist from all over the world to be able to study the same elements.
32. A model of a solution where water is the solvent and oxygen gas is the solute:
33. No. It is possible to have 100% "chemical free" water because it is impossible to have completely pure water when the atmospheric gases, nitrogen, oxygen, and carbon dioxide, always dissolve in water. Even with the expensive process of distillation, it is still impossible to have 100% "chemical free" water.
34. Water is composed of two hydrogen atoms and one oxygen atom and water's physical property is a liquid. However, the elements that make up water are not a liquid. The physical property of both hydrogen and oxygen is a gas.
Lab Report: Water Testing
LAB REPORT
Water Testing
6/25/13
Charlie, Ben, Georgie
Summer 2013
Dr. Foreman
PURPOSE:
The purpose of this lab was to learn how to test the presence of
dissolved ions in different kinds of water including; distilled water,
reference solution, control solution, tap water, and natural water (ocean
water). And by doing this, we learned a lot about ions.
ABSTRACT:
In this experiment, we tested for the presence of different ions through tests that confirms the presence of ions, otherwise known as confirming tests. My team and I preformed four different tests to test for the presence of dissolved calcium, iron, chloride, and sulfate ions. We would test for the these ions by using 5 five different solutions: distilled water, reference solution, control solution, tap water, and natural water (ocean water). By testing for ions with these different types of solutions, my team and I hoped we would be able to see which types of solutions had a change in color or had an appearance of participate, or even no reaction at all. These tests are important to help us reach a conclusion to what contaminates our water, and can therefore go through the necessary processes needed to purify the water.
PROCEDURE:
- Calcium Ion Test:
- Place 20 drops of distilled water into a 24-well plate
- Place 20 drops of reference solution (Ca Chloride) into a 24-well plate
- Place 20 drops of control solution into a 24-well plate
- Place 20 drops of Tap water into a 24-well plate
- Place 20 drops of Natural water into a 24-well plate
- Add 3 drops of sodium carbonate test solution to each of the wells
- Record your observations in your data table
- Decide whether CA2+ cations are present and record your results
- Discard the contents of the well plates as directed by your teacher
- Iron Ion Test
- Place 20 drops of distilled water into a 24-well plate
- Place 20 drops of reference solution (Ferric Nitrate) into a 24-well plate
- Place 20 drops of control solution into a 24-well plate
- Place 20 drops of Tap water into a 24-well plate
- Place 20 drops of Natural water into a 24-well plate
- Add one to two drops of potassium thiocyanate test reagent to each of the wells
- Record your observations in your data table
- Decide whether Fe3+ cations are present and record your results
- Discard the contents of the well plates as directed by your teacher
- Chloride Ion Test
- Place 20 drops of distilled water into a 24-well plate
- Place 20 drops of reference solution (Ca Chloride) into a 24-well plate
- Place 20 drops of control solution into a 24-well plate
- Place 20 drops of Tap water into a 24-well plate
- Place 20 drops of Natural water into a 24-well plate
- Add 3 drops of silver nitrate test reagent to each of the wells
- Record your observations in your data table
- Decide whether Cl- anions are present and record your results
- Discard the contents of the well plates as directed by your teacher
- Sulfate Ion Test
- Place 20 drops of distilled water into a 24-well plate
- Place 20 drops of reference solution (Ferric Nitrate) into a 24-well plate
- Place 20 drops of control solution into a 24-well plate
- Place 20 drops of Tap water into a 24-well plate
- Place 20 drops of Natural water into a 24-well plate
- Add 3 drops of barium carbonate test solution to each of the wells
- Record your observations in your data table
- Decide whether SO4^2- anions are present and record your results
- Discard the contents of the well plates as directed by your teacher
RESULTS:
After my team and I preformed 4 different tests to test for the presence of dissolved calcium, iron, chloride, and sulfate ions, we were able to see which types of solutions had a ion appearance. And after filling out our data table, we are able to conclude that in the calcium ion test there was no presence of calcium ions in the distilled water, tap water, or control solution; however, in the reference solution and the natural water there was a presence of theses ions. In the iron ion test, we were able to conclude that there was no presence of iron ions in the distilled water, the natural water, and the reference solution; however, in the control solution and the tap water there was a presence of these iron ions. In the chloride ion test, we concluded that there was no presence of chloride ions in distilled water and natural water, on the other hand, there was a presence of these chloride ions in the reference solution, the control solution, and tap water. Finally, in our sulfate ion test, we were able to conclude that there was no presence of sulfate ions in the distilled water and the tap water; therefore, there was a presence of these sulfate ions in the reference solution, the control solution, and the natural water. Overall, I think my group and I worked better than we ever had. We were more organized than before, by assigning specific jobs to each other, and by listening to what we each had to say. Although we were confused on what to do in the beginning, we worked hard with each other, and created a better understanding of what we had to do to make this lab successful.
Data Table: Calcium Ion Test:
Data Table: Iron Ion Test:
Data Table: Chloride Ion Test:
Data Table: Sulfate Ion Test:
Class results:
For the class results, we focused mainly on the results from the control solution and the ocean water. From these results we created a histogram, with all of the classes data together. From the histogram, I was able to conclude that many groups had the same results, and many groups had a variety of results. Most groups for their calcium test got no appearance of calcium ion for there control substance, and a variety got yes for ion appearance in there ocean water. For the iron test, most groups got no for ion appearance in the control substance, and all groups got no for ion appearance for ocean water. For the chloride test, there was a variety for the appearance or no appearance of the chloride ion in the control substance; however in the ocean water, most groups did have the appearance of the chloride ion. For the sulfate test, all groups got the same results, which was there was an appearance of sulfate ions in both the control solution and the ocean water. I think the variety of results has to do with how well each group rinsed their wells after each test, and how much of each solution they used.
Lab Questions #1-4:
1. A reference solution is a solution of known compositions used as a comparison. There were both reference and blank solutions in each test to give us something to compare our results to.
2. Some possible problems associated with the use of qualitative tests are that these tests only test for the presence or absence of a substance, not the amount of the substance. Also a problem that may arise from this kind of test is that sometimes water may appear pure, and but can actually be contaminated.
3. These tests cannot absolutely confirm the absence of an ion because sometimes a negative test might mean an ion is not present, or the ion is present in such a low amounts.
4. Our observations might have changed if we had not cleaned our wells or stirring rods thoroughly after each test because our observations would have been falsely impacted by the previous substances; therefore, giving us false results.
Monday, June 24, 2013
Blog Question
How does testing water help us?
Testing water is helpful because it can help us test for what may be polluting water, therefore, destroying our water supply. It will help us determine if there are any dissolved harmful materials in our water. By testing water, we can stop the spread of foul water, and therefore, stop people from getting sick.
Testing water is helpful because it can help us test for what may be polluting water, therefore, destroying our water supply. It will help us determine if there are any dissolved harmful materials in our water. By testing water, we can stop the spread of foul water, and therefore, stop people from getting sick.
ISBS (p.51) #19-24
6/24/13
19.
a) carbon: 6 protons, 6 electrons
b) aluminum: 13 protons, 13 electrons
c) lead: 82 protons, 82 electrons
d) chlorine: 17 protons, 17 electrons
20.
a) sulfer: 16 protons, 18 electrons: No
b) iron: 26 protons, 24 electrons: No
c) silver: 47 protons, 47 electrons: Yes
d) iodine: 53 protons, 54 electrons: No
21.
a) anion
b) neutral
c) neutral
d) cation
e) cation
22.
a) gaining electrons
b) neither
c) neither
d) losing electrons
e) losing electrons
23.
a) hydrogen with 1 proton and 1electron: H
b) sodium with 11 protons and 10 electrons: Na+
c) chlorine with 17 protons and 18 electrons: Cl-
d) aluminium with 13 protons and 10 electrons: Al+
24.
a) K+ and I-: Potassium iodine: KI
b) Ca2+ and S2-: Calcium sulfide: CaS
c) Fe3+ and Br-: Iron bromide: Fe3+(Br-)3: (Fe)(Br)3
d) Ba2+ and OH-: Barium hydroxide: Ba2+(OH-)2: (Ba)(OH)2
e) NH4+ and PO43-: Ammonium phosphate: (NH4+)3 + PO43-: (NH4)3(PO4)
f)Al3+ and O2-: aluminum oxide: (Al3)2 + (O2-)3: (Al)2(O)3
19.
a) carbon: 6 protons, 6 electrons
b) aluminum: 13 protons, 13 electrons
c) lead: 82 protons, 82 electrons
d) chlorine: 17 protons, 17 electrons
20.
a) sulfer: 16 protons, 18 electrons: No
b) iron: 26 protons, 24 electrons: No
c) silver: 47 protons, 47 electrons: Yes
d) iodine: 53 protons, 54 electrons: No
21.
a) anion
b) neutral
c) neutral
d) cation
e) cation
22.
a) gaining electrons
b) neither
c) neither
d) losing electrons
e) losing electrons
23.
a) hydrogen with 1 proton and 1electron: H
b) sodium with 11 protons and 10 electrons: Na+
c) chlorine with 17 protons and 18 electrons: Cl-
d) aluminium with 13 protons and 10 electrons: Al+
24.
a) K+ and I-: Potassium iodine: KI
b) Ca2+ and S2-: Calcium sulfide: CaS
c) Fe3+ and Br-: Iron bromide: Fe3+(Br-)3: (Fe)(Br)3
d) Ba2+ and OH-: Barium hydroxide: Ba2+(OH-)2: (Ba)(OH)2
e) NH4+ and PO43-: Ammonium phosphate: (NH4+)3 + PO43-: (NH4)3(PO4)
f)Al3+ and O2-: aluminum oxide: (Al3)2 + (O2-)3: (Al)2(O)3
Sunday, June 23, 2013
Blog Question
What interested you the most in the first week? What will you remember later?
What interested me the most in the first week was the distillation process. I thought it was really interesting to learn how dissolved particles in water, can just be heated, allowing theses dissolved particle (salt in water) to be separated from water. Therefore, making the water pure. I learned how the water is heated, causing it to turn into water vapor, and then it will fall as water again, furthermore, separating the water from the salt. I also liked seeing how foul water can activate electrical current and pure water cannot. The distillation process is something I will remember later.
What interested me the most in the first week was the distillation process. I thought it was really interesting to learn how dissolved particles in water, can just be heated, allowing theses dissolved particle (salt in water) to be separated from water. Therefore, making the water pure. I learned how the water is heated, causing it to turn into water vapor, and then it will fall as water again, furthermore, separating the water from the salt. I also liked seeing how foul water can activate electrical current and pure water cannot. The distillation process is something I will remember later.
A.7 (p.20 -21) #1-7 (only do #1 and 2)
6/21/13
1. 3,043 + 2,052.1 + 2,096.9 = 7,192 L used by my household in three days.
2. One member of my household used about 600 L of water on Day One.
3. A histogram of the class average per person.
4. The rang of the average daily personal water use within my class is 1,493 L.
5. The mean value of the class data is 600 L and the median value of the class date is 579. The mean is the better representation of the data set because it is the average, therefore, it is more accurate than the median.
6. The class average of the class is 600 L, and the average water use in the United States is 370 L. Some reasons I can propose to explain the difference between my value and the national average value is that the houses in our class may have bigger yards, and therefore need more water. Another reason why our water value is higher than the national average is because we live in a dessert, which requires us to use water all year long on our yards. Unlike places where is snows and rains.
7. The average of the class value is closer to the national average than the average water use by person daily because the average class water use is 579L and the national average use is 370 L. The reason I can give for why the class average is closer to the national average is because there are a lot of people in each of the calculations.
Question: What do our results tell us?
-Our results tell us that the more people the lower the average per person is, and the less people the more the average per person is. It also tells us the that different activities in the house can either cause the water average to rise, or sometimes even lessen.
14.
a) Models i, ii, iv, and vi represent models
b) Models iii, iv, and v represent compounds
15. The two pieces of information a chemical formula provides is one it is a "word" that represents chemical substances. And two, it contains chemical symbols that represents each element, and subscripts that indicates how many atoms of an element are present in the substance.
16.
a) Phosphoric acid (H3PO4): three hyrdrogen atoms, one phosphorus atom, and four oxygen atoms.
b) Sodium hydroxide (NaOH): one sodium atom, one oxygen atom, and one hydrogen atom.
c) Sulfer dioxide (SO2): one sulfer atom, and two oxygen atoms
17.
a) H2 + Cl2 --> 2 HCl
b) 2 H2O2 --> 2 H2O + O2
c) 17a: two hydrogen atoms + two chlorine atoms --> 2 hydrogen and chlorine. 17b: 2 compounds of hydrogen and oxygen atoms --> 2 compounds of two hydrogen atoms and one oxygen atom + two oxygen atoms.
18. Chemical equations that represents word equations
a) NaHCO3 + HCl --> NaCl + H20 + CO2
b) C6H12O6 + 6 O2 --> 6 CO2 + 6 H2O
1. 3,043 + 2,052.1 + 2,096.9 = 7,192 L used by my household in three days.
2. One member of my household used about 600 L of water on Day One.
3. A histogram of the class average per person.
5. The mean value of the class data is 600 L and the median value of the class date is 579. The mean is the better representation of the data set because it is the average, therefore, it is more accurate than the median.
6. The class average of the class is 600 L, and the average water use in the United States is 370 L. Some reasons I can propose to explain the difference between my value and the national average value is that the houses in our class may have bigger yards, and therefore need more water. Another reason why our water value is higher than the national average is because we live in a dessert, which requires us to use water all year long on our yards. Unlike places where is snows and rains.
7. The average of the class value is closer to the national average than the average water use by person daily because the average class water use is 579L and the national average use is 370 L. The reason I can give for why the class average is closer to the national average is because there are a lot of people in each of the calculations.
Question: What do our results tell us?
-Our results tell us that the more people the lower the average per person is, and the less people the more the average per person is. It also tells us the that different activities in the house can either cause the water average to rise, or sometimes even lessen.
ISBS (p.50-52) #13-18
13. Model of a molecular compound made up of two atoms of A and one atom
of D
14.
a) Models i, ii, iv, and vi represent models
b) Models iii, iv, and v represent compounds
15. The two pieces of information a chemical formula provides is one it is a "word" that represents chemical substances. And two, it contains chemical symbols that represents each element, and subscripts that indicates how many atoms of an element are present in the substance.
16.
a) Phosphoric acid (H3PO4): three hyrdrogen atoms, one phosphorus atom, and four oxygen atoms.
b) Sodium hydroxide (NaOH): one sodium atom, one oxygen atom, and one hydrogen atom.
c) Sulfer dioxide (SO2): one sulfer atom, and two oxygen atoms
17.
a) H2 + Cl2 --> 2 HCl
b) 2 H2O2 --> 2 H2O + O2
18. Chemical equations that represents word equations
a) NaHCO3 + HCl --> NaCl + H20 + CO2
b) C6H12O6 + 6 O2 --> 6 CO2 + 6 H2O
Thursday, June 20, 2013
B.5 (p.33) #1-3
6/20/13
1. A homogeneous mixture composed of three different gaseous elements:
2. The kind of matter the model represents is a heterogeneous mixture because the compounds aren't disrupted evenly throughout the model, instead it is split between the bottom and the top.
3.
A. Mixture of gaseous elements X and Z:
B. Two- atom compound of X and Z:
C. Four- atom compound of X and Z:
D. Solution composed of a solvent that is a two-atom compound of L and R, and a solute that is a composed of two atoms of element D and one atom of element T:
1. A homogeneous mixture composed of three different gaseous elements:
2. The kind of matter the model represents is a heterogeneous mixture because the compounds aren't disrupted evenly throughout the model, instead it is split between the bottom and the top.
3.
A. Mixture of gaseous elements X and Z:
B. Two- atom compound of X and Z:
C. Four- atom compound of X and Z:
D. Solution composed of a solvent that is a two-atom compound of L and R, and a solute that is a composed of two atoms of element D and one atom of element T:
Wednesday, June 19, 2013
ISBS (p.50) #1-12
6/19/13
1. A physical property is a property that can be observed and measured without changing the chemical makeup of the substance.
2. Three physical properties of water are density, freezing point, and boiling point.
3. The density of solid water compares to the density of liquid water because liquid water has a higher density then solid water.
4. A setting where I might observe water as a solid, a liquid, and a gas all at the same time would be if i was in Lake Tahoe. Because the lake would demonstrate water as a liquid, the mountain with snow would demonstrate water as a solid, and when the sun hits the snow it causes the ice to turn into water vapor, and therefore a gas.
5. Heterogeneous mixture is a mixture that isn's the same throughout. For example, foul water because the coffee grounds sink to the bottom and are not distributed evenly throughout the liquid. Homogeneous mixtures are the same through out. For example, salt water because the salt particles become uniformly mingled with the particles of water.
6. I need to know the density to know if either gasoline or water will be on top.
7. A. colloid
B. colloid
C. solution
D. solution
E. suspension
F. colloid
8. The air in the room demonstrates a colloid effect because it is demonstrating a Tyndall effect , which is when a scattering of light can pass through a clear object.
9.
10. The red liquid material is a colloid because in a long period of time no particles settled to the bottom. If the liquid was a solution, then the particles would have settled to the bottom over a period of time. And if the liquid was a suspension then there would have been large particle, and those would have for sure sunk to the bottom over a period of time. So, therefore, the liquid has to be a colloid.
11. A "substance" is an element or a compound. It is a material with a uniform, definiate composition, and distinct properties. Two examples of a substance are salt and water.
12. A. compound
B. element
C. compound
D. element
E. compound
F. compound
G.
1. A physical property is a property that can be observed and measured without changing the chemical makeup of the substance.
2. Three physical properties of water are density, freezing point, and boiling point.
3. The density of solid water compares to the density of liquid water because liquid water has a higher density then solid water.
4. A setting where I might observe water as a solid, a liquid, and a gas all at the same time would be if i was in Lake Tahoe. Because the lake would demonstrate water as a liquid, the mountain with snow would demonstrate water as a solid, and when the sun hits the snow it causes the ice to turn into water vapor, and therefore a gas.
5. Heterogeneous mixture is a mixture that isn's the same throughout. For example, foul water because the coffee grounds sink to the bottom and are not distributed evenly throughout the liquid. Homogeneous mixtures are the same through out. For example, salt water because the salt particles become uniformly mingled with the particles of water.
6. I need to know the density to know if either gasoline or water will be on top.
7. A. colloid
B. colloid
C. solution
D. solution
E. suspension
F. colloid
8. The air in the room demonstrates a colloid effect because it is demonstrating a Tyndall effect , which is when a scattering of light can pass through a clear object.
9.
10. The red liquid material is a colloid because in a long period of time no particles settled to the bottom. If the liquid was a solution, then the particles would have settled to the bottom over a period of time. And if the liquid was a suspension then there would have been large particle, and those would have for sure sunk to the bottom over a period of time. So, therefore, the liquid has to be a colloid.
11. A "substance" is an element or a compound. It is a material with a uniform, definiate composition, and distinct properties. Two examples of a substance are salt and water.
12. A. compound
B. element
C. compound
D. element
E. compound
F. compound
G.
Tuesday, June 18, 2013
Blog Response
Blog Response
Weigh in: Which is worse? Water shortages vs. Water pollution. Know the chemical properties of water that bear on these two issues. Debate online and in class. Inform yourself first. Have an informed opinion, Be able to argue either sides.
-Water shortage is worse because water is essential for human existance. Even with dirty water, you can purify it. But without any water, there is no water to be purified.
-Water pollution is worse because dirty water can cause many diseases or infections in the body; therefore, sometimes leading to death.
*The chemical properties of water are H2O
Weigh in: Which is worse? Water shortages vs. Water pollution. Know the chemical properties of water that bear on these two issues. Debate online and in class. Inform yourself first. Have an informed opinion, Be able to argue either sides.
-Water shortage is worse because water is essential for human existance. Even with dirty water, you can purify it. But without any water, there is no water to be purified.
-Water pollution is worse because dirty water can cause many diseases or infections in the body; therefore, sometimes leading to death.
*The chemical properties of water are H2O
1SAS (p.23) #3, 4, 5, 6, 7
6/18/13
3.
a. Manufacture of the filter paper is an indirect
water use because you had to water the tree that the paper came from, you
didn’t directly use the water in the machine
b. Premoistening of the sand and gravel are direct
water uses because you have to use water from the sink as a the source for
wetting the particles
c. The use of water to cool the distillation
apparatus is a direct water use because you use water from the sink to cool the
substance.
4. To purify water means to remove as much of the solid
particle from liquid and putting the liquid through various steps of filtration
that at some point the water can be used to wash your hands or brush your
teeth.
5. Three techniques for purifying water are oil- water
separation, sand filtration, and charcoal adsorption and filtration.
6. What was removed from the foul- water sample in each step
was in the oil- water separation; oil was removed from the sample. In the sand
filtration process, solid particles were removed from the sample. And from the
charcoal and filtration process, other dissolved particles were removed from
the sample giving it a clear look.
7.
a. The procedure used in the foul-water laboratory
investigation could not convert seawater suitable for drinking because salt
particles are so small that when they are mixed with water, the salt dissolves
in the water.
b. The additional purification steps needed to make seawater
suitable for drinking would be to heat the substance allowing the water to
evaporate, leaving only the salt.
A.8 (p.22) #1-4
1. Three water uses that I could do without are washing cars,
washing clothes, and washing pets.
2. One activity that I could not do without would be washing my
hair/hands.
3. The tasks I could reduce my water use is by taking shorter
showers and baths. And by washing my hands for a shorter amount of time.
4.
a. The activities that I could use such impure water are
when I am washing my parents car, washing the windows, or watering indoor or
outdoor plants.
b. The prior uses this water can be taken would be from
washing your teeth and hands.
Subscribe to:
Comments (Atom)